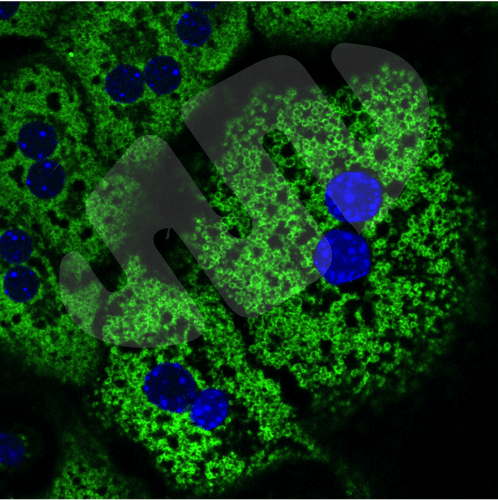
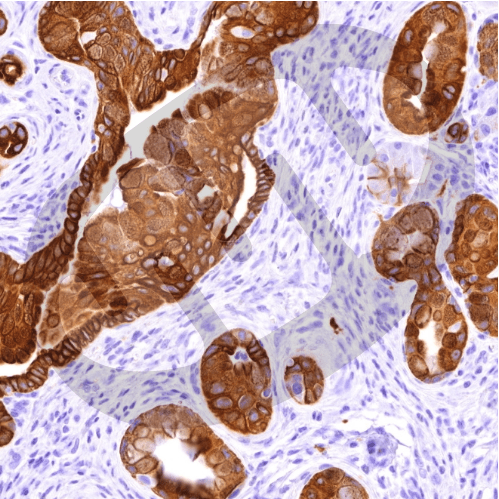
We use cutting-edge approaches in cell and molecular biology, systems biology, novel mouse models, and human patient samples to ask some of the most important questions about how mitochondria control tissue homeostasis, and how mitochondrial dysfunction contributes to cancer progression and metastasis. For example, we examine how BNIP3-dependent mitophagy is essential for proper metabolic zonation in the liver across the hypoxic gradient making up the liver lobule and how loss of BNIP3 promotes hepatic steatosis and liver cancer. This tumor-suppressive role of BNIP3 involves coordinated turnover of lipid droplets with mitochondria at the autolysosome in a process we have termed “mitolipophagy.”
In addition, we address how the BNIP3-related protein, BNIP3L (or NIX) which is also a mitochondrial cargo receptor, shares mitophagy functions with BNIP3 but we are increasingly focused on the divergent properties of BNIP3 and NIX in cellular growth control in liver, pancreas and in breast cancer metastasis.
Our work indicates that while BNIP3 and NIX share functions in mitophagy in various tissues, they also possess unique growth control properties that explain how BNIP3 functions to suppress tumor growth while NIX acts as a tumor promoter.
Ongoing work is addressing these functions in breast cancer, hepatocellular carcinoma, pancreatic ductal adenocarcinoma (PDAC) and in muscle atrophy linked to cancer cachexia. In other areas, we are examining how defects in mitophagy can promote tumor responses to chemotherapy and radiotherapy.
Finally, we are examining how mitochondrial stress affects other signaling pathways in the cell.